COVID-19 updates part 2 - the vaccine
Vaccination:
This blogpost is a bit longer so we had best start with the punchline. When it is your turn please get vaccinated against COVID-19. This includes people with Crohn’s disease and ulcerative colitis and other immune-mediated inflammatory diseases. This is true regardless of medical therapy. The vaccines are highly effective and have a good safety profile. We need as many adults as possible to get vaccinated to protect those most vulnerable from the virus, including a very small minority who won’t be able to get vaccinated. Then life can get back to normal. We need to vaccinate a lot of people across the world. This will take multiple vaccines. It will be the core global mission of 2021 and a huge triumph if we can come together to deliver this.
Next is the WOW:
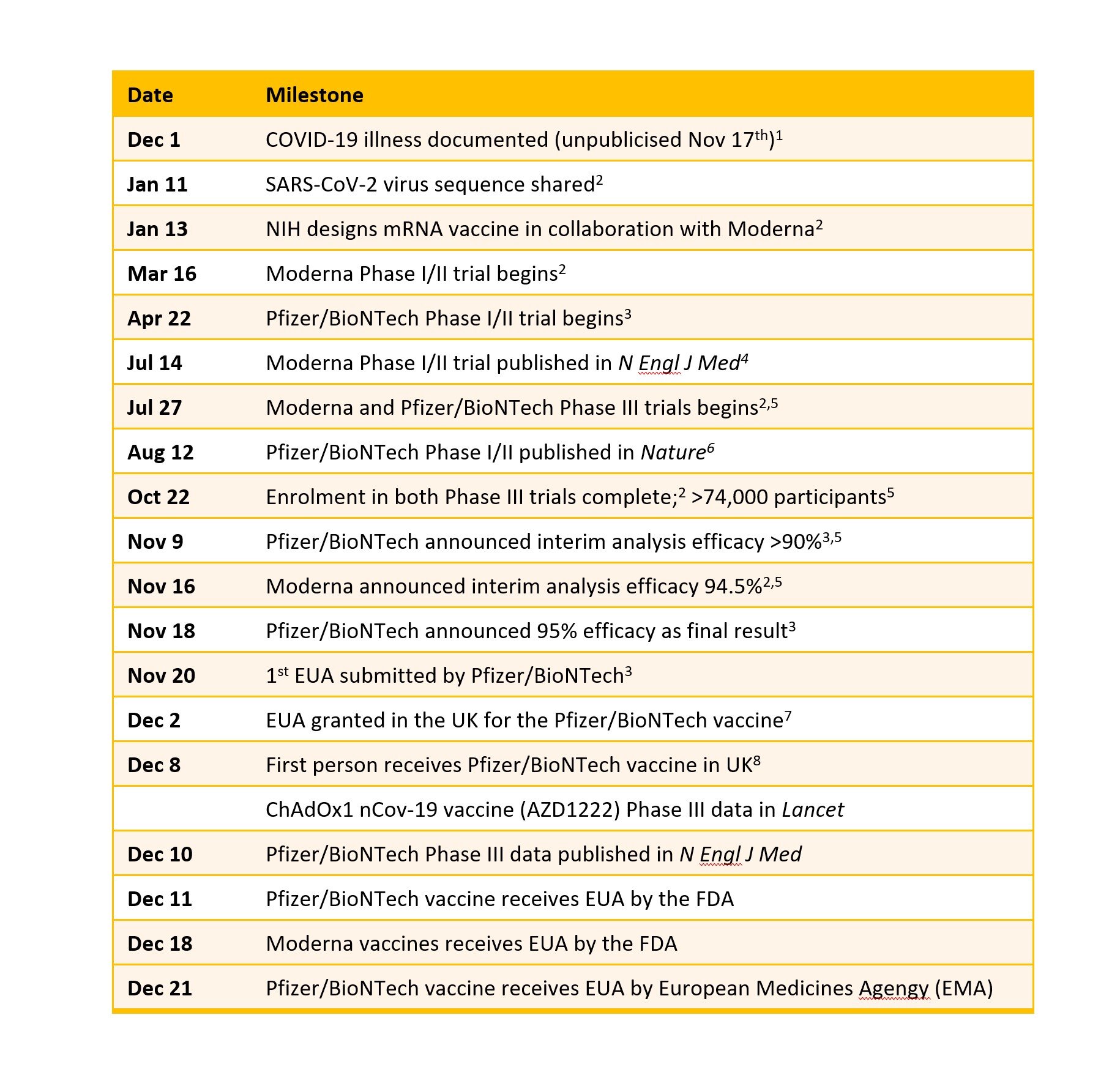
You will have heard it already by now but it is worth reiterating. Going from novel coronavirus to not one but three effective vaccines in the same calendar year is truly staggering. It will go down as one of humanities greatest achievements. In the spring many of us said that science would be our exit strategy from the pandemic. I don’t know anyone who seriously believed it would come this quickly. Usually it takes 10-15 years to develop and approve an effective vaccine. In that situation all key events happen sequentially – this minimises financial risk. For SARS-CoV-2 many key processes have run in parallel shortening timelines to 10-12 months. How is this possible? Simply put – money. This now seems obvious but it is worth reflecting that until very recently we did not know if it would be possible to produce any effective vaccines against this new coronavirus. For a brilliant detailed technical review article I recommend Florian Kramer’s piece in Nature - https://www.nature.com/articles/s41586-020-2798-3
Three vaccines have to date shown to be effective in the key phase 3 clinical trials – those from Pfizer/BioNTEch, Moderna and Astra/Oxford. Each of these trials involves tens of thousands of people who are randomised to get either the COVID-19 vaccine or a placebo. A period of time follows during which both study participants and investigators are blinded to who has had vaccine or placebo. During this follow-up period some people get symptomatic COVID-19 disease. At a certain point, enough people have reached this primary endpoint for the investigators to unblind those that got COVID-19. They then add up the numbers in each arm of the study and do some stats. Then the moment of truth. Must have been and exciting series of zoom calls when the results were revealed! This is the key graph from the Pfizer vaccine study.
How do the vaccines work?
The Pfizer/BioNTech and Moderna vaccines are both based on mRNA technology which now looks to be a highly effective and safe strategy. The mRNA molecules are wrapped up in a microbubble of fat that enables them to survive the intra-muscular injection and then be taken up by a variety of cells. The Astra/Oxford vaccine uses a viral vector to get the key instructions into cells. In this case the vector is a chimpanzee adenovirus (the type that typically causes colds) which is replication deficient. None of these vaccines are live. All of these vaccines are targeting the spike protein that sticks out of the coronavirus and allow it to enter into cells. All three viruses require two doses of vaccine given 3-4 weeks apart. After each dose of vaccine your body produces some of the spike protein but none of the actual coronavirus. Our immune system then gets to work ultimately learning and remembering the spike protein. That means that if you come across SARS-CoV-2 your body will see the spike protein and then mount the necessary antibody and T cell response to get rid of not just this protein but the whole virus.
There are about 50 COVID-19 vaccines in clinical development with approximately another 160 in pre-clinical development. Here are the details of these 3 vaccines plus those in phase 3 trials from Janssen and Novovax (details from https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines).
Will the vaccines work against the new variant?
I wrote yesterday about the new variant B.1.1.17 (https://charlielees.com/blog/covid-19-updates-part-1-the-new-variant). Although we don’t know anything definitive yet, expert commentary and scientists at BioNTech have today stated that they expect the vaccines to work against the new variant.
What to expect?
I got my first dose of the Pfizer vaccine on 10th December. The injection was painless. I had a sore arm for 2 days afterwards. This is very common (about 70-80%) and more of an effect than a side effect! It really just means that the vaccine is working. Some people will get a headache and some will feel a bit tired afterwards. None of this should last more than a day or two. A couple of people have had a significant allergic reaction to the Pfizer vaccine in the UK. Both of them had a prior history of anaphylaxis such that they carried an adrenaline autoinjector (EpiPen) with them. This is very rare.
How many people have been vaccinated?
As of today (22nd December) approximately 2.4 million people across the world have received at least one dose of a COVID-19 vaccine (https://t.co/03pQ8rRViP?amp=1). This includes about half a million people in both the UK and the USA who have received their first dose of the Pfizer vaccine. The roll-out of the Moderna vaccine started yesterday in the USA having received FDA approval last week. The Astra/Oxford vaccine is currently under assessment by the UK regulator, the MHRA. A number of people in China (approx. 1m) and Russia have been vaccinated with different vaccines which are subject to different regulatory approvals. The chart shows the proportions per 100 people vaccinated – most of this is with the Pfizer vaccine.
Should you take the vaccine if you have an immune mediated inflammatory disease like Crohn’s disease or ulcerative colitis?
Yes – absolutely. This is my strong recommendation to all my patients with inflammatory bowel disease. A statement to this effect from the British Society of Gastroenterology will be coming soon.
What if I take an immunosuppressant medicine?
The vaccines will remain very low risk for people with IBD regardless of what medicine(s) they are taking. It is possible that the vaccines will be slightly less effective for people taking certain medicines like anti-TNF agents (infliximab and adalimumab). If this is the case then these people may need an extra dose. But we do not know this yet and it is not a reason to defer getting vaccinated. We and others will study this so we know the answer in plenty of time.
Other questions?
I hope I have covered the key topics here but there will be very many additional questions. There is a really superb page on the Crohn’s and Colitis UK website which addresses these key questions - https://www.crohnsandcolitis.org.uk/news/latest-coronavirus-vaccine-for-people-with-crohns-or-colitis. You can also watch the panel Q&A I hosted two weeks ago here - https://youtu.be/7Cf-KrCiiDw.
And finally:
We have to remember to observe physical distancing, mask wearing and hand washing now more than ever. This is very important to ensure we allow the vaccination programme every chance of success and for life to return to normal as quickly as possible.